How to Calculate Specific Heat: 6 Steps
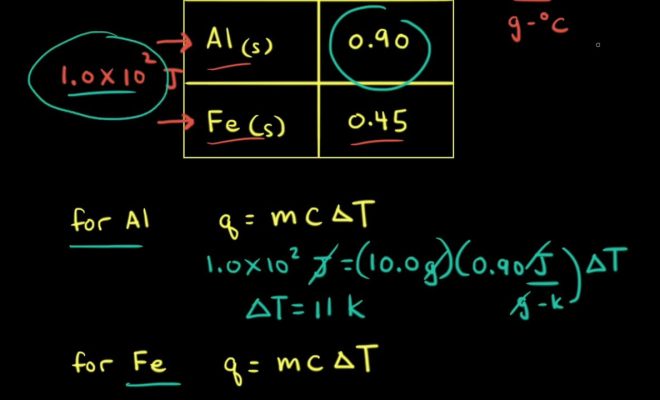
Introduction:
Specific heat is an essential concept in thermodynamics, as it helps us better understand how heat is absorbed or released by a substance during changes in temperature. It is the amount of heat required to increase the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin). In this article, we will present six steps to calculate specific heat using simple methods.
1. Identify the variables:
Before starting any calculations, it’s crucial to understand the variables involved – the mass of the substance (m), its change in temperature (ΔT), and the absorbed or released heat energy (q). Keep in mind that these variables should be measured in consistent units (e.g., kilograms for mass and Joules for heat energy).
2. Determine the mass of the substance:
To calculate specific heat, you need to know the mass of the sample. This can typically be determined by weighing the substance with a scale, but sometimes other methods may be necessary depending on the material you’re working with.
3. Measure or calculate temperature change:
Using a thermometer, measure both initial (T1) and final temperatures (T2) after heating or cooling occurs. Then find the change in temperature ΔT, which is simply: ΔT = T2 – T1.
4. Calculate heat energy transfer:
When measuring specific heat capacity, you’re often dealing with a controlled experiment where external factors influencing heat transfer are well documented. In this case, you can use a calorimeter to measure the absorbed or released heat energy directly (q). Alternatively, you can calculate q based on known values and formulas like q = mcΔT.
5. Plug values into specific heat formula:
The specific heat formula is: c = q / (m * ΔT), where c represents specific heat capacity (units commonly used include J/(kg·K) or J/(kg·°C)). Plug in your measured values for q, m, and ΔT, and solve for c.
6. Analyze the results:
After completing the calculations, you should have a value for specific heat capacity (c) within an acceptable range of error. Compare your results with published or known values to ensure your calculation is accurate.
Conclusion:
Calculating specific heat can help us better understand how substances react to changes in temperature and enhance our knowledge of fundamental thermodynamic principles. By following these six steps, you’ll be on your way to accurately determining specific heat capacities of various materials.