How to Calculate Percent Yield: A Comprehensive Guide
Introduction
In the world of chemistry, percent yield is a crucial concept for gauging the efficiency of a reaction. It is a measure of how much product was actually obtained compared to the theoretical amount expected based on stoichiometry. Understanding how to calculate percent yield is essential for students, researchers, and professionals alike. This article will guide you through the process of calculating percent yield step-by-step.
Step 1: Write a balanced chemical equation
The first step in calculating percent yield is to write a balanced chemical equation for the reaction of interest. In this equation, the coefficients in front of each molecule represent the stoichiometric ratios, which determine the quantities of reactants consumed and products generated.
Step 2: Determine theoretical yield
The theoretical yield is the maximum amount of product that can be formed in a reaction when all limiting factors are taken into account – such as limiting reactants and conversion rates. To calculate this value:
1. Identify the limiting reactant – this is the reactant that will be completely consumed first and therefore determines how much product can be produced.
2. Using stoichiometry, calculate how many moles of product can be generated from this limiting reactant.
3. Convert moles to mass by multiplying moles by the molecular weight (or molar mass) of the product.
Step 3: Obtain actual yield
The actual yield is the real amount of product obtained from an experiment or process and is usually determined by direct measurement or analysis.
Step 4: Calculate percent yield
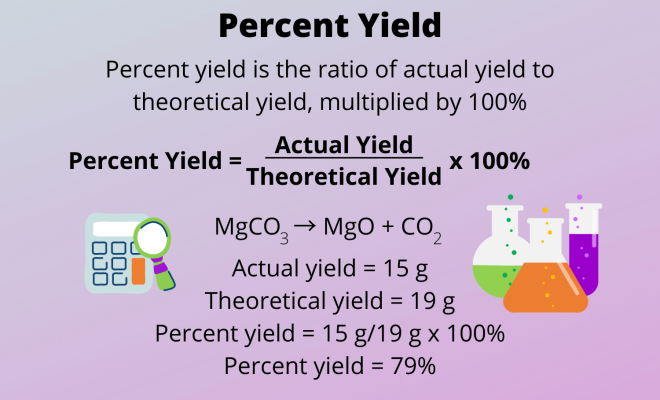
To calculate percent yield, use the following formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100%
where Actual Yield is the mass of product obtained from the experiment, and Theoretical Yield is the expected mass based on stoichiometry.
Example:
Consider a reaction where A + B → C and let’s assume we’ve conducted an experiment based on this reaction. We have the following details:
– Balanced equation: 2A + 3B → C
– Actual yield of C: 25 g
– Limiting reactant: A (assume we found this after comparing moles of A and B)
– Amount of A present: 30 g
– Molecular weights (or molar mass): A = 10 g/mol, B = 15 g/mol, C = 45 g/mol
Now we can calculate the percent yield using these details:
1. Convert the mass of A to moles: (30 g) / (10 g/mol) = 3 moles of A
2. Determine product formation from moles of A: From the balanced equation, 2A = C; Hence, 1 mole of C is produced from every 2 moles of A; We have 3 moles of A, so (3/2) moles of C can be formed.
3. Calculate theoretical yield: [(3/2) moles] × [45 g/mol] = 67.5 g
4. Calculate percent yield: (25 g / 67.5 g) × 100% ≈ 37%
In this example, the percent yield for C is approximately 37%.
Conclusion
Calculating percent yield is a crucial skill for understanding and optimizing chemical reactions. By following these steps, you can determine how efficient a reaction is and make necessary adjustments to improve the process or troubleshoot potential issues. Always remember to write a balanced chemical equation, identify the limiting reactant and use stoichiometry to round off your calculations accurately.